Dee Griffin, UNL-Great Plains Veterinary Educational Center, Clay Center, NE (DGriffin2@UNL.Edu)
What does the FDA’s December 2013 Feed Antibiotic Control Announcement Mean to Cattle Producers?
Perhaps the best description is the FDA announced the final step in a process that started almost two decades ago. The bottom line is this final step offers more for livestock producers than it is thought to take away.
When did the FDA really start working with antibiotic resistance issues in livestock?
Two decades ago the FDA CVM (Center for Veterinary Medicine) recognized antibiotic resistance was making it harder to properly treat livestock. Drugs like Oxytet, PenG, Tylan, and Erythromycin had become less and less effective for treating diseases such as pneumonia. Working with congress, the FDA-CVM, livestock organizations and veterinary organization got legislation passed with a long legal name and hence goes by the more user friendly name of AMDUCA (Animal Medicinal Drug Use Clarification Act). This legislation, for the first time, allowed livestock to receive the proper dose of an animal drug regardless of the dose prescribed on the label and was referred to as “Extra Label Drug Use” (ELDU). It applied to over the counter (OTC) medications like Penicillin G. The label legally only allowed 1CC per 100 lbs (CWT). This is an ineffective dose, but doses higher than that would likely cause a violative drug residue. Causing a violative drug residue is a criminal offense; not a good situation for livestock producers or their vets! AMDUCA, in part provided a mechanism for better estimating the proper withdrawal if a drug required ELDU to be effective. AMDUCA has played an integral part in not only improving the proper antibiotic dosing in livestock, but also helped pave the way to better understanding of how to properly assign withdrawal times for drugs in which the ELDU applied. The statistically calculated random residue sampling program in the U.S. verifies we have a ZERO violative residue rate in all foods derived from meat, milk, and poultry.
What was the FDA’s next step on dealing with antibiotic resistance concerns?
AMDUCA was followed by almost a decade of work between the FDA and Center for Disease Control (CDC) trying to better understand the origin and trends of antibiotic resistance. The first part of this effort was CDC’s “National Antibiotic Resistance Monitoring System” (NARMS) which began in 1996. The system targeted enteric (coliform) bacteria such as Salmonella, E. coli, and Campylobacter. Of particular interest was the potential for antibiotic resistance in these bacteria that might be transferred from animals to humans via food. Yearly, thousands of samples of enteric bacteria from hundreds of laboratories across the U.S. (coast to coast and border to border) are submitted to the CDC NARMS laboratories for evaluation.
The evaluation led FDA and CDC scientists to develop a FDA CVM Guidance for Industry document (GFI) that would change the way antibiotics for animals would be approved in the future. The document was named GFI #152 and laid out a nine cell antibiotic resistance concern grid.
The grid (figure 1 http://go.unl.edu/4vum) categorized antibiotics and their relationship to antibiotic resistance that could affect humans as; “critically important”, “important”, or “not important” to humans. Additionally, the grid categorized the potential for antibiotic resistance to develop based on the duration the bacteria would be exposed to an antibiotic. The document indicated there was minimal potential for bacteria to develop resistance if the exposure to the antibiotic was less than seven days. But indicated a serious concern for development of antimicrobial resistance if the bacteria were exposed to the antibiotic for longer than 21 days. Antibiotics fed to livestock often fit into the “bacteria exposed for greater than 21 days” category and were therefore of more concern.
Additionally, the decision was made to never approve an antibiotic in the future for “production purposes” such as “improved growth or gain” or for “improved feed efficiency” or for “improved reproductive efficiency”.
During this time frame many very effective antibiotics such as Draxxin, Excede, Resflor, Zactran, and Zuprevo have been approved for individual use in livestock. But new approvals for antibiotics that could be used in feed halted due to the concerns for potential antibiotic resistance of enteric bacteria such as Salmonella, E. coli, and Campylobacter that may be transferred to humans via food and potentially cause disease.
How to reopen the FDA approval process for antibiotics that can be fed to livestock for disease treatment, prevention, and/or control.
Livestock producers were stuck with the antibiotics that had been approved … nothing new was going to be available to help manage health problems in their livestock. It is important to remember we have very good products to control coccidia (Rumensin, Bovatec, and Deccox). We have chlortetracycline (CTC, Aureomycin) for treatment of scours caused by E. coli and pneumonia caused by Pasteurella multocida, and we have tylosin (Tylan) to control liver abscesses caused by Fusobacterium. But no new antibiotics for use in feed would be forth coming unless the FDA-CVM figured out a mechanism that the antibiotic will use WITHOUT jeopardizing the safe guards needed to protect humans.
In April 2012, the FDA-CVM announced a new Guidance for Industry named GFI #209. This document outlined the development of “Veterinary Feed Directives” (VFD) and represented a mechanism that could be used by the FDA to approve an antibiotic for use in livestock feed to “treat”, “prevent”, or “control” a “specific” disease causing bacteria in a “targeted” group of “affected animals” or a group of animals at a “high risk” of developing a disease caused by a “specific” bacteria.
The idea was not new. Veterinary Feed Directives had been successfully used in swine operations. The expansion of the VFD to include cattle had many obstacles to overcome. Not the least of which is how to make a medication readily available when and where the medication is needed when a disease event occurs. And additionally, how can the public be assured the medications are being used in accordance with FDA legal regulations?
The first VFD medication approved was Pulmotil for the treatment of pneumonia in swine and cattle. The pharmaceutical company that makes Pulmotil has worked hard through their network of distributors to insure the medication has been available when and where it is needed. Public assurance of the responsible use of the medication comes through the documentation required by the FDA when the medication is used. Yes, the paper work is a hassle, but in the first year of VFD availability the FDA has streamlined some required documentation. Additionally, through free enterprise, a company stepped up and now offers electronic management of the documentation. Their service, now unwritten by the pharmaceutical company, improves speed, accuracy, and decreases errors.
What is next?
The success of the FDA-CVM VFD effort to make available an antibiotic for livestock disease management AND document the ability to safe guard the public’s interest in decreasing the risk of antibiotic resistance development has led the FDA-CVM to the final step in eliminating unwarranted “production use” (improved growth, gain, efficiency) of antibiotics in livestock in which “bacteria are exposed for greater than 21 days” to the antibiotic and for which there is no medically warranted need.
On December 11, 2013 the FDA-CVM announced the GFI #213. This document is directed to pharmaceutical companies that have antibiotics that currently have “production use” labeling (improved growth, gain, efficiency). On December 12, 2013 an additional VFD document, 21CFR 514 and 558, was published in the Federal Register. This document outlines the specific steps pharmaceutical companies will take to have their antibiotic considered for being approved for use under the VFD requirement.
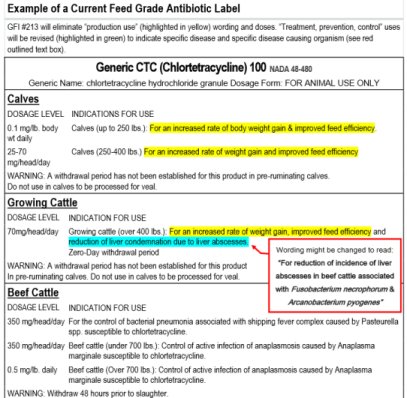
The FDA-CVM has made GFI #213 and associated VFD requirements available for evaluation and comment since early 2012 and therefore come as no surprise to pharmaceutical companies. On the same day GFI #213 was announced, two major pharmaceutical companies enthusiastically endorsed GFI #213. Companies that currently have antibiotics with “production use” have three years to follow the 21CFR 514 and 558 requirements to gain approved VFD labeling. In three years all production use labels will expire. Here is an example of a current label with GFI #213 indicated changes http://go.unl.edu/7iwo.
Why would a pharmaceutical company or livestock owner “enthusiastically endorse” the FDA-CVM announcement of GFI #213?
With the expiration of “production use” (improved growth, gain, efficiency) labeling, comes the opportunity for FDA approval of several new VFD medications. The one VFD made available thus far is superior for treatment of pneumonia than any antibiotic that has been available in the past and it seems very realistic that other, even more effective VFD antibiotics currently exist and are only an approval process away from being available for use under VFD requirements.
Livestock owners get the potential for new more effective medications that can be used in the feed to manage disease events and give up nothing. The “production use” (improved growth, gain, efficiency) labeling is decades out of date. There are long lists of theories about how antibiotics improved growth, gain, and efficiency, but nothing has ever stood the test of time and scrutiny better than “good health” as the mechanism for improved growth, gain, and efficiency. The data is lacking to support the use of antibiotics in feed for improved growth, gain, efficiency, so much so the National Cattlemen’s Beef Association (NCBA) technical and scientific advisor group in 2001 recommended the addition of an additional item on the NCBA’s “Antibiotic Prudent Use Guidelines” that read, “Sub-therapeutic Antibiotic Use is Discouraged: Antibiotics should be limited to prevent or control disease and should not be used if the principle intent is to improve performance.”
Bottom line
The downside is there will be paper work involved for using VFD medications. In time we will learn how to streamline the paperwork. Already, an enterprising company this last year figured out how to make managing VFD paperwork much easier.
A decade from now, if the antibiotic resistance has decreased we will all be glad we participated in helping the effort. If antibiotic resistance doesn’t change, the world will know the problem they blamed on us wasn’t of our making and we will likely have gotten a few new feed usage antibiotics that are much more effective than what we currently have available. It seems to me, perhaps a win-win for us.