Pocket Science: Exploring the 'What,' 'So what' and 'Now what' of Husker research
by Scott Schrage | University Communication
Welcome to Pocket Science: a glimpse at recent research from Husker scientists and engineers. For those who want to quickly learn the “What,” “So what” and “Now what” of Husker research.
What?
Any cook worth their salt knows that a dash of the stuff — which consists mostly of the compound sodium chloride — will dissolve when dropped into a pot of even room-temperature water.
But as a chemist who has spent decades researching how substances behave when confined to infinitesimal spaces, Nebraska’s Xiao Cheng Zeng also knows that what happens at the macroscale does not necessarily hold at the nanoscale.
So what?
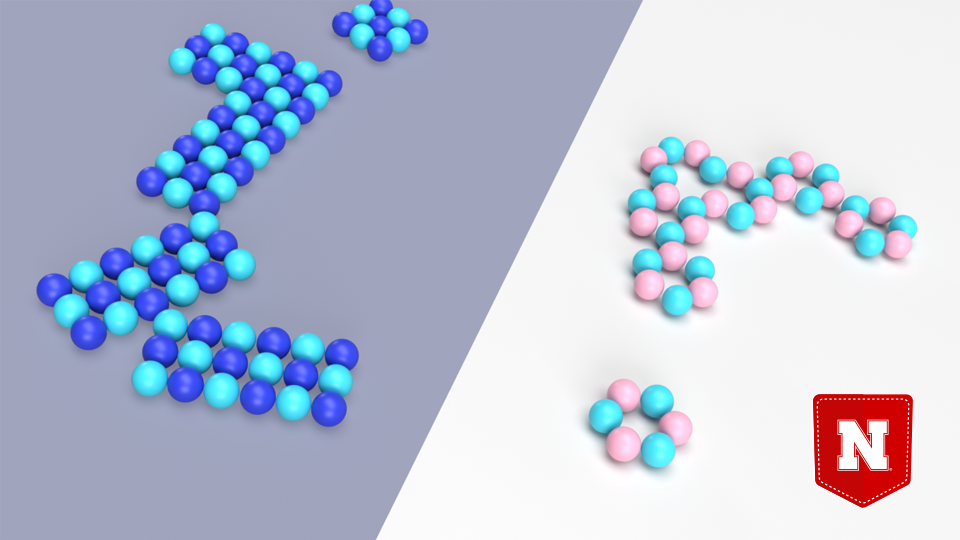
Zeng and his colleagues recently ran computer simulations to determine how sodium chloride and its salty cousin, lithium chloride, might respond when submerged in a nanoscopic stream of water bordered by two smooth, water-repellent walls.
Those simulations predicted something wildly counterintuitive. After initially dissolving in the water, the charged, randomly dispersed atoms of both sodium and lithium chloride would spontaneously reassemble into 2D layers, according to the simulations. In the case of sodium chloride, that layer would be identical to its solid, pre-dissolved state: a crystalline pattern of squares, with each sodium atom surrounded by four chlorine atoms, or vice versa. For lithium chloride, the layer would comprise hexagonal rings — three lithium atoms, three chlorine — or zigzagging chains of the atoms, or both.
Based on the team’s calculations, the unexpected behavior emerges partly because nanoscale confinement reduces the interaction strength between a charged atom — sodium, lithium or chlorine — and the water molecules that typically form a shell around it. That hydration shell normally keeps oppositely charged particles, such as sodium and chlorine, from reassembling after dissolving — but not when confined to a nanoscopic space, the researchers found.
Now what?
Zeng and his fellow computational chemists hope their predictions will encourage other researchers to conduct experiments that validate or challenge their simulations.
Those predictions might eventually inform the design of nanofluidic devices that transport charged atoms to recreate neuronal activity, Zeng said.
https://news.unl.edu/newsrooms/today/article/pinch-the-salt-dissolved-salt-can-reassemble-at-nanoscale-simulations-say/