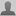Scientists model 'Lord of the Nanorings'
Released on 03/22/2005, at 12:00 AM
Office of University Communications
University of Nebraska–Lincoln
It's possible that no one gets more use out of the University of Nebraska-Lincoln's PrairieFire supercomputer, than Xiao Cheng Zeng and his collaborators.
In the past five years, they have used PrairieFire to model a list of previously unknown nanoscale substances and structures, from two-dimensional ice that shrinks when it freezes (the so-called "Nebraska" ice) to silicon nanotubes that behave like metals and four new kinds of one-dimensional ice crystals.
Now, they have modeled what they jokingly call the "Lord of the Nanorings," a ring of 20 boron atoms that is so stable that the rings can be stacked to create a tube of virtually any length with a diameter of a mere 2.6 nanometers (2.6 billionths of a meter).
The results of the experiments were announced in the Jan. 25 issue of the Proceedings of the National Academy of the Sciences. It was the fourth time in the last five years that research by Zeng and his team was published in one of the major international journals.
Zeng, Willa Cather professor of chemistry at UNL, said the boron nanoring, if it can be created in the laboratory, could open the door to the development of ultrasmall and ultralight radiation detectors, even smaller than the neutron-detection device that a team of UNL scientists and engineers produced three years ago. That hand-held device was built around a boron-carbide semiconductor diode that is smaller than a dime but can be used for locating hidden nuclear materials, monitoring nuclear weapons storage and other national security applications.
In fact, Zeng said, his decision to study boron clusters during a sabbatical last year on a prestigious Guggenheim Fellowship was in large part motivated by his collaboration with two members of that team, physicists Peter Dowben of UNL and Wei-ning Mei of the University of Nebraska at Omaha, in studying the formation of boron-carbide molecular clusters.
"I'm collaborating with Peter Dowben and Wei-ning Mei to study the most-stable boron-carbide cluster because of their interest in boron-carbide," Zeng said. "We have made some progress in that and certainly if we understand the small components of the boron-carbide clusters, we can help them make their device smaller and smaller."
In studying the growth patterns of atomic clusters on PrairieFire, Zeng and his team add one atom at a time to see when an element makes the transitions from one-dimensional (linear) to two-dimensional (flat) and three-dimensional.
When they began their investigation of where boron makes its transition from flat to three-dimensional, they were able to focus on the fairly narrow range of 16, 18 or 20 atoms. The upper estimate was based on Buckminster Fuller's prediction in the early 1980s that carbon forms perfect three-dimensional cages (the so-called "buckyballs") at 20 atoms. Zeng and his team reasoned that since boron and carbon are both insulators and sit next to each other on the periodic table (atomic Nos. 5 and 6), they might have similar growth patterns. The lower range was known because a team of physicists at Washington State University in Pullman, led by Professor Lai-Sheng Wang, had established in laboratory experiments that boron clusters up to 15 atoms remained flat.
"I asked my graduate student, Satya Bulusu, to check into this transition problem, and I have a senior student, Soohaeng Yoo, who had earlier created a computer program to study silicon clusters, and we were able to use it to study boron clusters, too," Zeng said. "Bulusu first checked 16 atoms and it was flat. Then we became a little bit impatient and we jumped over to 20 atoms because of carbon's transition to the buckyball at 20.
"We used PrairieFire for about three months running. Patiently, we waited three months and we found this three-dimensional ring -- and it's a beautiful structure. At the time that we discovered it, the movie, 'The Lord of the Rings,' was out and we joked that this ring is the 'Lord of the Nanorings.'"
Zeng and his team repeated the calculation at 18 atoms, but didn't find a stable three-dimensional cluster. Zeng said clusters of 17 and 19 atoms weren't tested because an odd number would not allow perfect ring symmetry.
To see if the "Lord of the Nanorings" could be created in a lab, Zeng and his team turned to Wang, one of world's leading experimenters in boron clusters, and whom Zeng came to know as a result of his Guggenheim Fellowship. Experiments by Wang's group, however, could only produce the ring structure by adding an electron to the 20 boron atoms. Otherwise, what they saw was a flat structure, a result that was disappointing but not discouraging to Zeng.
"Right now there are two ways to see this nanoring," he said. "One is that you have to synthesize it in a lab, like carbon 20 has been synthesized, and that is a challenge to experimenters. Another is to go down to a lower temperature."
Zeng said the experiments at Washington State were done at room temperature. At temperatures close to absolute zero (minus 273 degrees Celsius), where nature is much less chaotic, he said he thinks the ring structure would stand out. "It's by far the most stable," he said.
CONTACT: Xiao Cheng Zeng, Cather Professor, Chemistry, (402) 472-9894