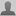Scientists create new form of matter that can dent diamonds
Released on 08/16/2012, at 1:00 PM
Office of University Communications
University of Nebraska–Lincoln


What do you get when you take buckyballs, soak them in a particular solvent and crush them under the pressure of more than 300,000 atmospheres?
The obvious answer is a bunch of crushed buckyballs. But a team of scientists that included University of Nebraska-Lincoln chemist Xiao Cheng Zeng has found that by using the right solvent at the right pressure, they created a new form of matter that they termed an "ordered amorphous carbon cluster." It's so hard it can dent diamonds, the hardest known substance.
Like diamonds, buckyballs (technically buckminsterfullerenes) are made of carbon. They're a well-ordered, cage-like structures of 60 carbon atoms that look remarkably like soccer balls. When the scientists smashed them, they lost their cage-like structure, as expected. What wasn't expected was what they turned into.
"It's a new form of matter not seen before," said Zeng, Ameritas University Professor of Chemistry at UNL. "The buckyballs originally are ordered, but if we crush them, it's an ordered amorphous carbon cluster. They become a mess, but they are still in a long-range order.
"And it turns out this new form of matter is super hard. It can indent diamonds."
The discovery was announced in a paper published in the Aug. 16 issue of the international journal Science.
The scientists infused the buckyballs with a solvent with the chemical formula of C8H10 (eight carbon atoms and 10 hydrogen atoms), an aromatic hydrocarbon based on benzene ("aromatic" meaning the atoms can share electrons).
Using a device called a diamond anvil cell, lead author Lin Wang of the Carnegie Institution of Washington in Argonne, Ill., subjected the buckyballs to steadily increasing pressures. Below approximately 30 gigapascals (nearly 300,000 atmospheres), the buckyballs bounced back to their normal shape after decompression. Above 32 gigapascals, however, the cages completely collapsed and transformed into amorphous clusters, but remarkably maintained their long-range order after decompression.
Subsequent X-ray tests by Wang, using the Advanced Photon Source at Argonne, and by Bingbin Liu, using the National Synchrotron Light Source at Brookhaven National Laboratory in Upton, N.Y., measured and confirmed the structure.
The new material's hardness was confirmed when the experimental team found indentations on the diamond anvils used in their experiments.
"The unique aspect of this experiment is the solvent the team used with the buckyballs before they crushed them, it was the crucial trick in making this new form of matter," Zeng said. "When they added the high pressure, the solvent molecules were still intact and separated the buckyballs, preventing them from forming polymers. The balls were highly damaged, but the entire system was still in ordered structure."
Zeng also said the specific solvent used was crucial. He said a team of scientists in Sweden tried a similar experiment several years ago, but used C8H8 as the solvent and didn't get the super-hard ordered amorphous carbon cluster.
The laboratory experiments and tests were further confirmed after Zeng and his postdoctoral researcher, Hui Li, the lead author in the computational study, used some 1.4 million computer hours performing large-scale quantum molecular dynamics simulations -- 900,000 hours at the Oak Ridge Leadership Computing Facility at Oak Ridge National Laboratory in Tennessee, and another 500,000 hours at UNL's Holland Computing Center. As a point of comparison, that's the rough equivalent of one top-of-the-line desktop computer running calculations continuously 24 hours a day for 160 years.
"The simulation gave us some important insight to this material, atomic insight, because in the experiment, it's very hard to see how the matter collapses," Zeng said. "With the super computers, we can monitor the pressure and then monitor the matter under high pressure at atomic scale under different pressures."
Zeng said there were several scientific motivations for the work, especially the never-ending search by materials scientists for new forms of matter. A second factor is the search for technologically useful matter. The fact that the new, super-hard form of matter preserves its high-pressure structure in ambient condition is very important for possible future practical applications.
The research was supported by the Office of Science, National Nuclear Security Administration, the Office of Basic Energy Sciences in the U.S. Department of Energy, and the National Science Foundation. Zeng and Li's portion of the research was also funded in part by the Nebraska Research Initiative.
It's the first time that work from Zeng's lab has been published in Science, the journal of the American Association for the Advancement of Science, but it's the 10th time in 11 years that his work has been published in one of the four highest-impact interdisciplinary journals (including twice this summer). His research previously appeared in the other three, Nature, the Proceedings of the National Academy of Sciences and Nature Communications.
Writer: Tom Simons, University Communications, 402-472-8514
News Release Contacts:
- xzeng1, Retiree UNL, Retirees
phone: 4024723501
Associated Media Files:
- Xiao Cheng Zeng (right) and postdoctoral researcher Hui Li with their computer-generated images of the creation of "ordered amorphous carbon clusters." (Photo: Craig Chandler, University Communications)
- Computer-generated illustration of buckyballs (pink) and the C8H10 solvent (blue) at normal atmospheric pressure (left); under approximately 200,000, 300,000, 330,000 and 400,000 atmospheres (center column); and returned to normal pressure (right column). At 300,000 atmospheres or less, the buckyballs returned to their original shape. At 330,000 or more, however, they were transformed into "ordered amorphous carbon clusters," a new form of matter that is hard enough to dent diamonds.