By Becky Schuerman, Extension Domestic Water/Wastewater Associate
There are naturally occurring elements and minerals within Nebraska geology, and with that, it is not uncommon to find them in Nebraska’s groundwater. Calcium, magnesium, iron, manganese, fluoride, arsenic and uranium are among the elements found in Nebraska.
Over the coming months, I will spotlight each of these elements in the NEBLINE newsletter. While every day should be “Groundwater Day” in Nebraska, March 7–13, is National Groundwater Week this year. To kick off the celebration, this month’s featured elements are calcium and magnesium.
CALCIUM AND MAGNESIUM
Calcium and Magnesium are elements that make a water considered “hard.” They are common metallic elements found within the soils and rock formations throughout Nebraska’s geology. Neither have a health risk affiliated with them. They are generally each considered more of a nuisance element because of their potential to cause mineral build-up/scale layer within hot water heaters, water pipes, boilers and other plumbing fixtures. These minerals also cause poor performance of soap and detergents.
TREATING HARD WATER
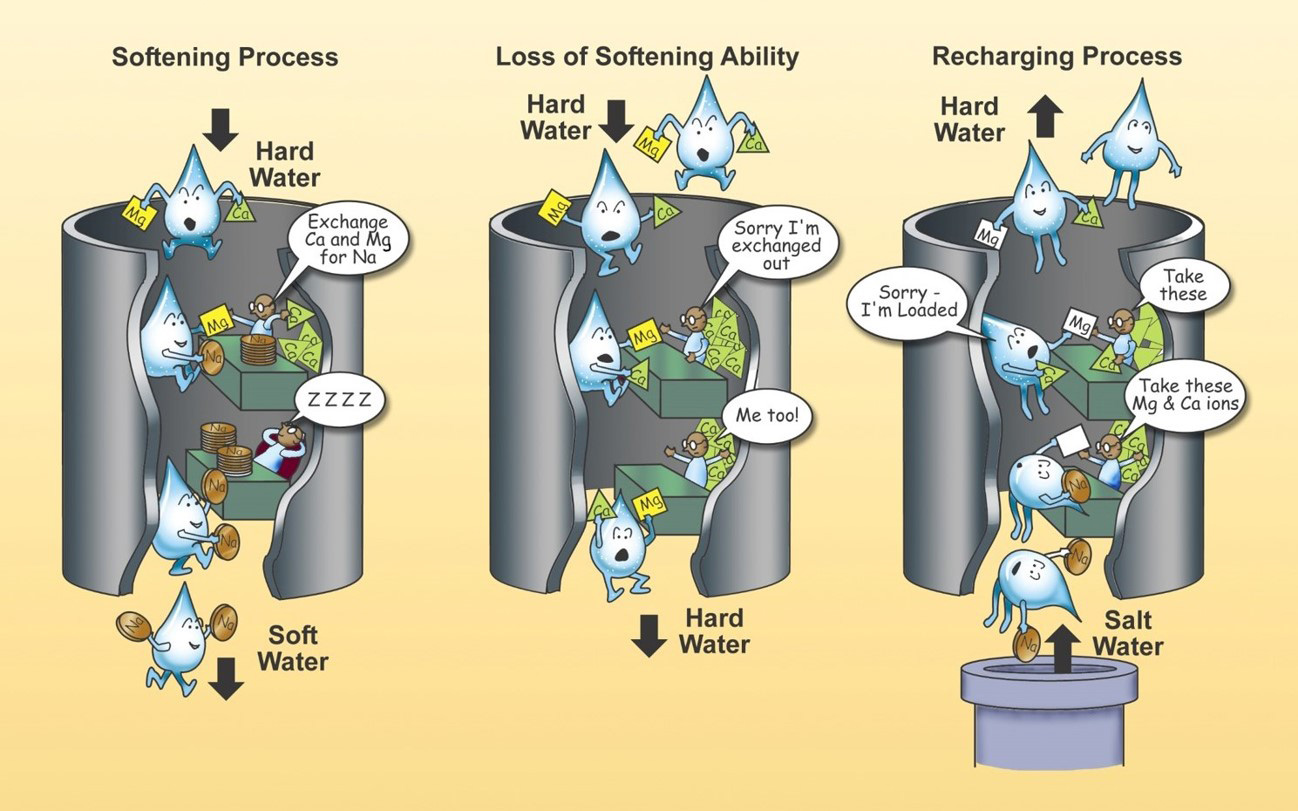
Hard water is most often treated with an ion exchange water softener that has sodium chloride or potassium chloride added to make the water less hard. People who have to watch their salt intake will want to avoid consuming water softened with sodium and either use a potassium chloride product or drink the hard water instead.
Most hard water contributes a small amount to the total calcium and magnesium required in one’s diet, but in some instances, it can be a major contributor. An ion exchange treatment system is considered point of entry (POE) treatment equipment, meaning that it treats all of the water coming into the home or building.
When looking to install a water softener, one should make sure that the unit meets NSF/ANSI 44 technical requirements. This approval means the unit has been rigorously tested and meets the public health compliance standards for residential water softeners that use ion exchange resin, regenerated with a sodium or potassium chloride product to reduce the hardness of water from a private well or public water supply. One can contact a reputable water quality professional in their area for water softening needs as well.
FOR MORE INFORMATION
For further information about hard water, see Nebraska Extension’s NebGuides at https://water.unl.edu/article/drinking-water/nebguides.
• “Drinking Water: Hard Water (Calcium and Magnesium)” (G1274)
• “Drinking Water Treatment: Water Softening (Ion Exchange)” (G1491)
****************************************
COMPLETE CAPTION FOR ILLUSTRATION
A simple overview of how the water softening process works. As hard water enters the water softener, it filters through a resin that is supersaturated with a sodium (Na) brine. The calcium (Ca) and magnesium (Mg) in the hard water attach to the resin beads and are exchanged for sodium (Na), thus making soft water for use throughout the home. Over time, the exchange resin becomes saturated with Ca and Mg and has to be regenerated with the Na brine solution so an effective water softening process can continue. (Graphic by Nebraska Extension)