By Becky Schuerman, Extension Domestic Water/Wastewater Associate
Many parts of Nebraska have hard water. Hard water has a high mineral content; hardness primarily refers to the amount of calcium (Ca) and magnesium (Mg) dissolved in the water. Hard water is not just a private well owner’s problem, it affects many municipal water users, too. While hard water does not present a health risk for the vast majority of the population, it is often a nuisance for homeowners across the state.
ION EXCHANGE TREATMENT
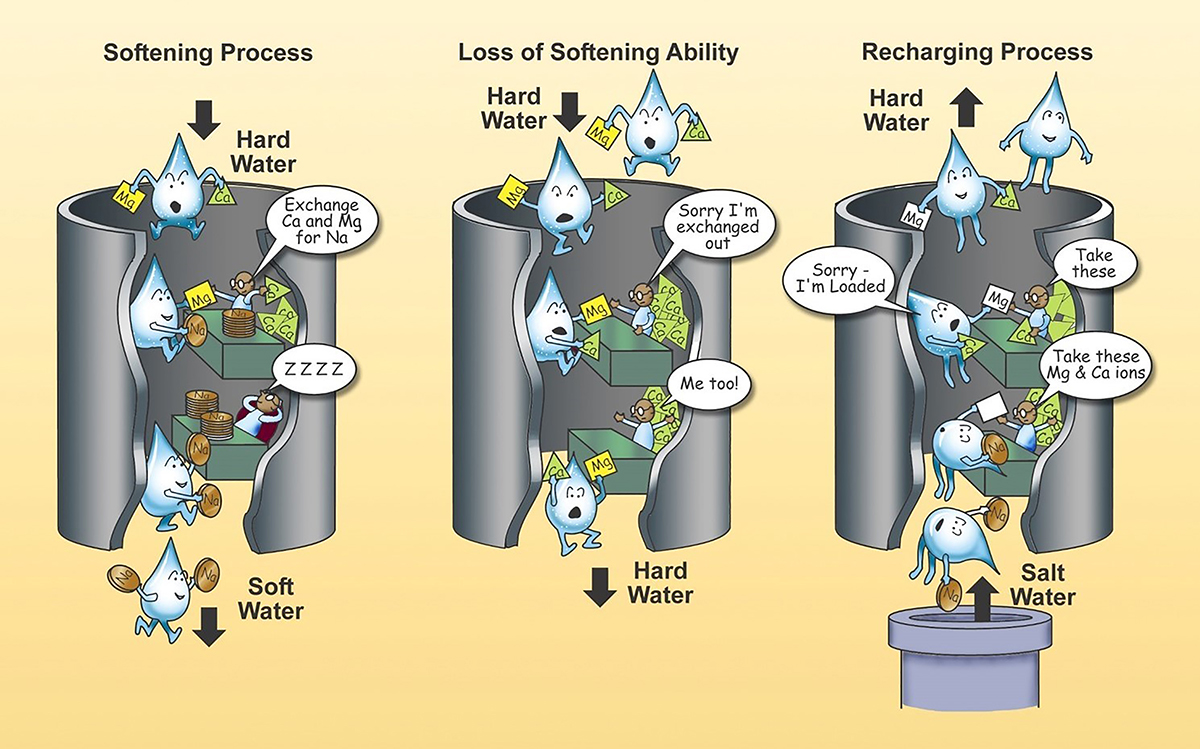
Hard water can be softened using an ion-exchange-softening process as illustrated in the figure above. Ion exchange involves removing the hardness ions Ca and Mg and replacing them with non-hardness ions, typically sodium supplied by dissolved sodium chloride salt or brine. A potassium chloride product can also be used within an ion exchange treatment system instead of a sodium chloride product. The softener contains a microporous exchange resin, usually sulfonated polystyrene beads that are supersaturated with sodium to cover the bead surfaces. As water passes through this resin bed, Ca and Mg ions attach to the resin beads and the loosely held sodium is released from the resin into the water. After softening a large quantity of hard water, the beads become saturated with Ca and Mg ions. When this occurs, the exchange resin must be regenerated, or recharged. To regenerate, the ion exchange resin is flushed with a salt or a potassium brine solution. The sodium ions in the salt or potassium brine solution are exchanged with the calcium and magnesium ions on the resin and excess calcium and magnesium is flushed out with waste water. Protocol has been established to assess the effectiveness of ion exchange water softeners (NSF/ANSI 44) technical requirements. An ion exchange treatment system is considered point-of-entry treatment equipment, meaning that it treats all of the water coming into the home or building.
EMERGING TECHNOLOGIES
There are a variety of devices that claim to manage hard water scale without the use of salt; using primarily emerging technologies such as electrochemical treatment, electrically induced precipitation and template-assistance crystallization. Manufacturers generally claim the devices utilize energy to alter the behavior of compounds or elements within the water so the Ca and Mg do not form a scale on pipes or plumbing fixtures. Some research supports the application of some of these devices in industrial applications for anti-scaling treatment. Currently, it is hard to predict which devices will work in all homes, given the high degree of variability in water quality and water use patterns. Standards and protocols for these no-salt softening devices are being developed and may offer testing and certification of these household products in coming years. These no-salt technologies offer consumers both the promise and problems that may occur with emerging technologies.
FOR MORE INFORMATION
For further information about hard water, see Nebraska Extension’s NebGuides at https://water.unl.edu/article/drinking-water/nebguides.
• “Drinking Water: Hard Water (Calcium and Magnesium)” (G1274)
• “Drinking Water Treatment: Water Softening (Ion Exchange)” (G1491)